Isoniazid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Hydra, Hyzyd, Isovit, others |
| Other names | isonicotinic acid hydrazide, isonicotinyl hydrazine, INH, INAH, INHA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682401 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Very low (0–10%) |
| Metabolism | liver; CYP450: 2C19, 3A4 inhibitor |
| Elimination half-life | 0.5–1.6h (fast acetylators), 2-5h (slow acetylators) |
| Excretion | urine (primarily), feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.195 |
| Chemical and physical data | |
| Formula | C6H7N3O |
| Molar mass | 137.142 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Isoniazid, also known as isonicotinic acid hydrazide (INH), is an antibiotic used for the treatment of tuberculosis.[4] For active tuberculosis, it is often used together with rifampicin, pyrazinamide, and either streptomycin or ethambutol.[5] For latent tuberculosis, it is often used alone.[4] It may also be used for atypical types of mycobacteria, such as M. avium, M. kansasii, and M. xenopi.[4] It is usually taken by mouth, but may be used by injection into muscle.[4]
History
[edit]First synthesis was described in 1912.[6] A. Kachugin invented the drug against tuberculosis under name Tubazid in 1949. Three pharmaceutical companies unsuccessfully attempted to patent the drug at the same time,[7] the most prominent one being Roche, which launched its version, Rimifon, in 1952.[8]
The drug was first tested at Many Farms, a Navajo community in Arizona, due to the Navajo reservation's tuberculosis problem and because the population had not previously been treated with streptomycin, the main tuberculosis treatment at the time.[9] The research was led by Walsh McDermott, an infectious disease researcher with an interest in public health, who had previously taken isoniazid to treat his own tuberculosis.[10]
Isoniazid and a related drug, iproniazid, were among the first drugs to be referred to as antidepressants.[11] Psychiatric use stopped in 1961 following reports of hepatotoxicity. Use against tuberculosis continued, as isoniazid's effectiveness against the disease outweighs its risks.[12]
It is on the World Health Organization's List of Essential Medicines.[13] The World Health Organization classifies isoniazid as critically important for human medicine.[14] Isoniazid is available as a generic medication.[4]
Medical uses
[edit]Tuberculosis
[edit]Isoniazid is often used to treat latent and active tuberculosis infections. In persons with isoniazid-sensitive Mycobacterium tuberculosis infection, drug regimens based on isoniazid are usually effective when persons adhere to the prescribed treatment. However, in persons with isoniazid-resistant Mycobacterium tuberculosis infection, drug regimens based on isoniazid have a high rate of failure.[15]
Isoniazid has been approved as prophylactic therapy for the following populations:
- People with HIV infection and a PPD (purified protein derivative) reaction of at least 5 mm induration
- Contacts of people with tuberculosis and who have a PPD reaction at least 5 mm induration
- People whose PPD reactions convert from negative to positive in a two-year period – at least 10 mm induration for those up to 35 years of age, and at least 15 mm induration for those at least 35 years old
- People with pulmonary damage on their chest X-ray that is likely to be due to healed tuberculosis and also have a PPD reaction at least 5 mm induration
- Injection drug users whose HIV status is negative who have a PPD reaction at least 10 mm induration
- People with a PPD of greater than or equal to 10 mm induration who are foreign-born from high prevalence geographical regions, low-income populations, and patients residing in long-term facilities[16][17]
Isoniazid can be used alone or in combination with Rifampin for treatment of latent tuberculosis, or as part of a four-drug regimen for treatment of active tuberculosis.[18] The drug regimen typically requires daily or weekly oral administration for a period of three to nine months, often under Directly Observed Therapy (DOT) supervision.[18]
Non-tuberculous mycobacteria
[edit]Isoniazid was widely used in the treatment of Mycobacterium avium complex as part of a regimen including rifampicin and ethambutol.[19] Evidence suggests that isoniazid prevents mycolic acid synthesis in M. avium complex as in M. tuberculosis[20] and although this is not bactericidal to M. avium complex, it greatly potentiates the effect of rifampicin. The introduction of macrolides led to this use greatly decreasing. However, since rifampicin is broadly underdosed in M. avium complex treatment, this effect may be worth re-investigating.[21]
Special populations
[edit]It is recommended that women with active tuberculosis who are pregnant or breastfeeding take isoniazid. Preventive therapy should be delayed until after giving birth.[22] Nursing mothers excrete a relatively low and non-toxic concentration of INH in breast milk, and their babies are at low risk for side effects. Both pregnant women and infants being breastfed by mothers taking INH should take vitamin B6 in its pyridoxine form to minimize the risk of peripheral nerve damage.[23] Vitamin B6 is used to prevent isoniazid-induced B6 deficiency and neuropathy in people with a risk factor, such as pregnancy, lactation, HIV infection, alcoholism, diabetes, kidney failure, or malnutrition.[24]
People with liver dysfunction are at a higher risk for hepatitis caused by INH, and may need a lower dose.[22]
Levels of liver enzymes in the bloodstream should be frequently checked in daily alcohol drinkers, pregnant women, IV drug users, people over 35, and those who have chronic liver disease, severe kidney dysfunction, peripheral neuropathy, or HIV infection since they are more likely to develop hepatitis from INH.[22][25]
Side effects
[edit]Up to 20% of people taking isoniazid experience peripheral neuropathy when taking daily doses of 6 mg/kg of body weight or higher.[26] Gastrointestinal reactions include nausea and vomiting.[16] Aplastic anemia, thrombocytopenia, and agranulocytosis due to lack of production of red blood cells, platelets, and white blood cells by the bone marrow respectively, can also occur.[16] Hypersensitivity reactions are also common and can present with a maculopapular rash and fever.[16] Gynecomastia may occur.[18]
Asymptomatic elevation of serum liver enzyme concentrations occurs in 10% to 20% of people taking INH, and liver enzyme concentrations usually return to normal even when treatment is continued.[27] Isoniazid has a boxed warning for severe and sometimes fatal hepatitis, which is age-dependent at a rate of 0.3% in people 21 to 35 years old and over 2% in those over age 50.[16][28] Symptoms suggestive of liver toxicity include nausea, vomiting, abdominal pain, dark urine, right upper quadrant pain, and loss of appetite.[16] Black and Hispanic women are at higher risk for isoniazid-induced hepatotoxicity.[16] When it happens, isoniazid-induced liver toxicity has been shown to occur in 50% of patients within the first 2 months of therapy.[29]
Some recommend that liver function should be monitored carefully in all people receiving it,[22] but others recommend monitoring only in certain populations.[27][30][31]
Headache, poor concentration, weight gain, poor memory, insomnia, and depression have all been associated with isoniazid use.[32] All patients and healthcare workers should be aware of these serious side effects, especially if suicidal ideation or behavior are suspected.[32][33][34]
Isoniazid is associated with pyridoxine (vitamin B6) deficiency because of its similar structure. Isoniazid is also associated with increased excretion of pyridoxine. Pyridoxal phosphate (a derivative of pyridoxine) is required for δ-aminolevulinic acid synthase, the enzyme responsible for the rate-limiting step in heme synthesis. Therefore, isoniazid-induced pyridoxine deficiency causes insufficient heme formation in early red blood cells, leading to sideroblastic anemia.[24]
Isoniazid was found to significantly elevate the in vivo concentration of GABA and homocarnosine in a single subject via magnetic resonance spectroscopy.[35]
Drug interactions
[edit]People taking isoniazid and acetaminophen are at risk of acetaminophen toxicity. Isoniazid is thought to induce a liver enzyme which causes a larger amount of acetaminophen to be metabolized to a toxic form.[36][37]
Isoniazid decreases the metabolism of carbamazepine, thus slowing down its clearance from the body. People taking carbamazepine should have their carbamazepine levels monitored and, if necessary, have their dose adjusted accordingly.[38]
It is possible that isoniazid may decrease the serum levels of ketoconazole after long-term treatment. This is seen with the simultaneous use of rifampin, isoniazid, and ketoconazole.[39]
Isoniazid may increase the amount of phenytoin in the body. The doses of phenytoin may need to be adjusted when given with isoniazid.[40][41]
Isoniazid may increase the plasma levels of theophylline. There are some cases of theophylline slowing down isoniazid elimination. Both theophylline and isoniazid levels should be monitored.[42]
Valproate levels may increase when taken with isoniazid. Valproate levels should be monitored and its dose adjusted if necessary.[40]
Mechanism of action
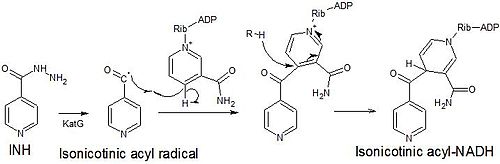
[edit]Isoniazid is a prodrug that inhibits the formation of the mycobacterial cell wall. Isoniazid must be activated by KatG, a bacterial catalase-peroxidase enzyme in Mycobacterium tuberculosis.[43] KatG catalyzes the formation of the isonicotinic acyl radical, which spontaneously couples with NADH to form the nicotinoyl-NAD adduct. This complex binds tightly to the enoyl-acyl carrier protein reductase InhA, thereby blocking the natural enoyl-AcpM substrate and the action of fatty acid synthase. This process inhibits the synthesis of mycolic acids, which are required components of the mycobacterial cell wall. A range of radicals are produced by KatG activation of isoniazid, including nitric oxide,[44] which has also been shown to be important in the action of another antimycobacterial prodrug pretomanid.[45]
Isoniazid is bactericidal to rapidly dividing mycobacteria, but is bacteriostatic if the mycobacteria are slow-growing.[46] It inhibits the cytochrome P450 system and hence acts as a source of free radicals.[47]
Isoniazid is a mild non-selective monoamine oxidase inhibitor (MAO-I).[48] It inhibits diamine oxidase more strongly. These two actions are possible explanations for its antidepressant action[49] as well as its ability to cause mania.[12]
Metabolism
[edit]Isoniazid reaches therapeutic concentrations in serum, cerebrospinal fluid, and within caseous granulomas. It is metabolized in the liver via acetylation into acetylhydrazine. Two forms of the enzyme are responsible for acetylation, so some patients metabolize the drug more quickly than others. Hence, the half-life is bimodal, with "slow acetylators" and "fast acetylators". A graph of number of people versus time shows peaks at one and three hours. The height of the peaks depends on the ethnicities of the people being tested. The metabolites are excreted in the urine. Doses do not usually have to be adjusted in case of renal failure.[citation needed]
Preparation
[edit]Isoniazid is an isonicotinic acid derivative. It is manufactured using 4-cyanopyridine and hydrazine hydrate.[50] In another method, isoniazid was claimed to have been made from citric acid starting material.[51]
It can in theory be made from methyl isonicotinate, which is labelled a semiochemical.
Brand names
[edit]Hydra, Hyzyd, Isovit, Laniazid, Nydrazid, Rimifon, and Stanozide.[52]
Other uses
[edit]Chromatography
[edit]Isonicotinic acid hydrazide is also used in chromatography to differentiate between various degrees of conjugation in organic compounds barring the ketone functional group.[53] The test works by forming a hydrazone which can be detected by its bathochromic shift.[citation needed]
Dogs
[edit]Isoniazid may be used for dogs, but there have been concerns it can cause seizures.[54]
References
[edit]- ^ "Isoniazid (Nydrazid) Use During Pregnancy". Drugs.com. 7 October 2019. Retrieved 24 January 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Drug and medical device highlights 2018: Helping you maintain and improve your health". Health Canada. 14 October 2020. Retrieved 17 April 2024.
- ^ a b c d e "Isoniazid". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 136. hdl:10665/44053. ISBN 9789241547659.
- ^ Saygin D, Tabib T, Bittar HE, Valenzi E, Sembrat J, Chan SY, et al. (1912-04-01). "Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension". Pulmonary Circulation (in German). 10 (1): 393–414. doi:10.1177/2045894020908782. PMC 7052475. PMID 32166015.
- ^ Rieder HL (April 2009). "Fourth-generation fluoroquinolones in tuberculosis". Lancet. 373 (9670): 1148–1149. doi:10.1016/S0140-6736(09)60559-6. PMID 19345815. S2CID 43789954.
- ^ "History". rocheusa.com. Roche USA. Archived from the original on 2007-12-12.
- ^ Jones DS (2002). "The health care experiments at Many Farms: the Navajo, tuberculosis, and the limits of modern medicine, 1952-1962". Bulletin of the History of Medicine. 76 (4): 749–790. doi:10.1353/bhm.2002.0186. PMID 12446978. S2CID 30166423.
- ^ Beeson PB (1990). "Walsh McDermott". Biographical Memoirs. Vol. 59. National Academies Press. pp. 282–307. doi:10.17226/1652. ISBN 978-0-309-04198-0.
- ^ Moncrieff J (June 2008). "The creation of the concept of an antidepressant: an historical analysis" (PDF). Social Science & Medicine. 66 (11): 2346–2355. doi:10.1016/j.socscimed.2008.01.047. PMID 18321627.
- ^ a b https://onlinelibrary.wiley.com/doi/full/10.1111/bdi.13272
- ^ Organization WH (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. 2019. hdl:10665/312266. ISBN 9789241515528.
- ^ Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D (February 2017). "Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis". The Lancet. Infectious Diseases. 17 (2): 223–234. doi:10.1016/S1473-3099(16)30407-8. PMID 27865891.
- ^ a b c d e f g "Isoniazid (package insert)". 30 March 2023.
- ^ "The Use of Preventive Therapy for Tuberculosis Infection in the United States – Recommendations of the Advisory Committee for Elimination of Tuberculosis". Morbidity and Mortality Weekly Report. 39 (RR-8): 9–12. May 18, 1990. Archived from the original on 2 March 2016. Retrieved 22 February 2016.
- ^ a b c Lewis SM, Dirksen SR, Heitkemper MM, Bucher L, Harding M (5 December 2013). Medical-surgical nursing : assessment and management of clinical problems (Ninth ed.). St. Louis, Missouri. ISBN 978-0-323-10089-2. OCLC 228373703.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Research Committee Of The British Thoracic Society (March 2001). "First randomised trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol". Thorax. 56 (3): 167–172. doi:10.1136/thorax.56.3.167. PMC 1758783. PMID 11182006.
- ^ Mdluli K, Swanson J, Fischer E, Lee RE, Barry CE (March 1998). "Mechanisms involved in the intrinsic isoniazid resistance of Mycobacterium avium". Molecular Microbiology. 27 (6): 1223–1233. doi:10.1046/j.1365-2958.1998.00774.x. PMID 9570407. S2CID 13764717.
- ^ van Ingen J, Egelund EF, Levin A, Totten SE, Boeree MJ, Mouton JW, et al. (September 2012). "The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment". American Journal of Respiratory and Critical Care Medicine. 186 (6): 559–565. doi:10.1164/rccm.201204-0682OC. PMID 22744719.
- ^ a b c d "Isoniazid tablet". DailyMed. 18 October 2018. Archived from the original on 13 March 2019. Retrieved 24 January 2020.
- ^ Bothamley G (2001). "Drug treatment for tuberculosis during pregnancy: safety considerations". Drug Safety. 24 (7): 553–565. doi:10.2165/00002018-200124070-00006. PMID 11444726. S2CID 10479433.
- ^ a b Steichen O, Martinez-Almoyna L, De Broucker T (April 2006). "[Isoniazid induced neuropathy: consider prevention]". Revue des Maladies Respiratoires. 23 (2 Pt 1): 157–160. doi:10.1016/S0761-8425(06)71480-2. PMID 16788441.
- ^ Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. (October 2006). "An official ATS statement: hepatotoxicity of antituberculosis therapy". American Journal of Respiratory and Critical Care Medicine. 174 (8): 935–952. doi:10.1164/rccm.200510-1666ST. PMID 17021358. S2CID 36384722.
- ^ Alldredge B (February 12, 2013). Applied Therapeutics. Lippincott Williams & Wilkins. ISBN 9781609137137.
- ^ a b "Latent Tuberculosis Infection: A Guide for Primary Health Care Providers". cdc.gov. Center for Disease Control. Archived from the original on 25 March 2016. Retrieved 25 March 2016.
- ^ Trevor, A. & Katzung, B. (2013). Katzung & Trevor's Pharmacology: examination & board review (10th ed., p. 417). New York. McGraw-Hill Medical, Lange.
- ^ "Isoniazid UpToDate". Archived from the original on 2015-10-25.
- ^ "Treatment of Tuberculosis – Guidelines (4th ed.)" (PDF). who.int. World Health Organization. Archived (PDF) from the original on 4 April 2016. Retrieved 25 March 2016.
- ^ Joint T (July 1998). "Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Joint Tuberculosis Committee of the British Thoracic Society". Thorax. 53 (7): 536–548. doi:10.1136/thx.53.7.536. PMC 1745276. PMID 9797751.
- ^ a b Alao AO, Yolles JC (September 1998). "Isoniazid-induced psychosis". The Annals of Pharmacotherapy. 32 (9): 889–891. doi:10.1345/aph.17377. PMID 9762376. S2CID 73122253.
- ^ Iannaccone R, Sue YJ, Avner JR (February 2002). "Suicidal psychosis secondary to isoniazid". Pediatric Emergency Care. 18 (1): 25–27. doi:10.1097/00006565-200202000-00008. PMID 11862134. S2CID 31383347.
- ^ Pallone KA, Goldman MP, Fuller MA (February 1993). "Isoniazid-associated psychosis: case report and review of the literature". The Annals of Pharmacotherapy. 27 (2): 167–170. doi:10.1177/106002809302700205. PMID 8439690. S2CID 28637999.
- ^ Landheer K, Prinsen H, Petroff OA, Rothman DL, Juchem C (June 2020). "Elevated homocarnosine and GABA in subject on isoniazid as assessed through 1H MRS at 7T". Analytical Biochemistry. 599: 113738. doi:10.1016/j.ab.2020.113738. PMID 32302606. S2CID 215809029.
- ^ Murphy R, Swartz R, Watkins PB (November 1990). "Severe acetaminophen toxicity in a patient receiving isoniazid". Annals of Internal Medicine. 113 (10): 799–800. doi:10.7326/0003-4819-113-10-799. PMID 2240884.
- ^ Burk RF, Hill KE, Hunt RW, Martin AE (July 1990). "Isoniazid potentiation of acetaminophen hepatotoxicity in the rat and 4-methylpyrazole inhibition of it". Research Communications in Chemical Pathology and Pharmacology. 69 (1): 115–118. PMID 2218067.
- ^ Fleenor ME, Harden JW, Curtis G (June 1991). "Interaction between carbamazepine and antituberculosis agents". Chest. 99 (6): 1554. doi:10.1378/chest.99.6.1554a. PMID 2036861.
- ^ Baciewicz AM, Baciewicz FA (September 1993). "Ketoconazole and fluconazole drug interactions". Archives of Internal Medicine. 153 (17): 1970–1976. doi:10.1001/archinte.153.17.1970. PMID 8357281.
- ^ a b Jonville AP, Gauchez AS, Autret E, Billard C, Barbier P, Nsabiyumva F, et al. (1991). "Interaction between isoniazid and valproate: a case of valproate overdosage". European Journal of Clinical Pharmacology. 40 (2): 197–198. doi:10.1007/BF00280078. PMID 2065702. S2CID 22218366.
- ^ Bass JB, Farer LS, Hopewell PC, O'Brien R, Jacobs RF, Ruben F, et al. (May 1994). "Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention". American Journal of Respiratory and Critical Care Medicine. 149 (5): 1359–1374. doi:10.1164/ajrccm.149.5.8173779. PMID 8173779.
- ^ Höglund P, Nilsson LG, Paulsen O (February 1987). "Interaction between isoniazid and theophylline". European Journal of Respiratory Diseases. 70 (2): 110–116. PMID 3817069.
- ^ Suarez J, Ranguelova K, Jarzecki AA, Manzerova J, Krymov V, Zhao X, et al. (March 2009). "An oxyferrous heme/protein-based radical intermediate is catalytically competent in the catalase reaction of Mycobacterium tuberculosis catalase-peroxidase (KatG)". The Journal of Biological Chemistry. 284 (11): 7017–7029. doi:10.1074/jbc.M808106200. PMC 2652337. PMID 19139099.
- ^ Timmins GS, Master S, Rusnak F, Deretic V (August 2004). "Nitric oxide generated from isoniazid activation by KatG: source of nitric oxide and activity against Mycobacterium tuberculosis". Antimicrobial Agents and Chemotherapy. 48 (8): 3006–3009. doi:10.1128/AAC.48.8.3006-3009.2004. PMC 478481. PMID 15273113.
- ^ Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, et al. (November 2008). "PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release". Science. 322 (5906): 1392–1395. Bibcode:2008Sci...322.1392S. doi:10.1126/science.1164571. PMC 2723733. PMID 19039139.
- ^ Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, et al. (October 2009). "Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig". The Journal of Infectious Diseases. 200 (7): 1136–1143. doi:10.1086/605605. PMID 19686043.
- ^ Harvey RA, Howland RD, Mycek MJ, Champe PC (2006). Harvey RA, Champe PC (eds.). Pharmacology. Vol. 864 (4th ed.). Lippincott Williams & Wilkins. ISBN 9780781741187.
- ^ Judd FK, Mijch AM, Cockram A, Norman TR (1994). "Isoniazid and antidepressants: is there cause for concern?". International Clinical Psychopharmacology. 9 (2): 123–125. doi:10.1097/00004850-199400920-00009. PMID 8056994.
- ^ Healy D (1998). The Psychopharmacologists. Vol. 2. A Hodder Arnold Publication. pp. 132–4. ISBN 978-1-86036-010-7.
- ^ William Andrew Publishing (2008). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Norwich, NY: Elsevier Science. pp. 1968–1970. ISBN 9780815515265.
- ^ Baizer MM, Dub M, Gister S, Steinberg NG (July 1956). "Synthesis of isoniazid from citric acid". Journal of the American Pharmaceutical Association. 45 (7): 478–480. doi:10.1002/jps.3030450714. PMID 13345683.
- ^ "Drugs@FDA". fda.gov. United States Food and Drug Administration. Archived from the original on 14 August 2012. Retrieved 22 August 2016.
- ^ Saygin D, Tabib T, Bittar HE, Valenzi E, Sembrat J, Chan SY, et al. (1959). "Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension". Pulmonary Circulation. 10 (1): 102–105. doi:10.1021/ac60145a020. PMC 7052475. PMID 32166015.
- ^ Sykes JE (2013). Canine and Feline Infectious Diseases (E-Book). Elsevier Health Sciences. p. 425. ISBN 978-0323241946 – via Google Books.
Further reading
[edit]- Romero JA, Kuczler FJ (February 1998). "Isoniazid overdose: recognition and management". American Family Physician. 57 (4): 749–752. PMID 9490997. Archived from the original on 2011-11-01. Retrieved 2005-12-13.
External links
[edit] Media related to Isoniazid at Wikimedia Commons
Media related to Isoniazid at Wikimedia Commons